Thalidomide - Contergan®
Annotated Collection of Links
Preface:
Thalidomide (also known under the brand names Contergan® in Germany and Kevadon® in Canada and the United States) became infamous in the early 1960's in the context of one of the biggest drug disasters of recent history. The substance with the chemical name (±)-N-(2,6-Dioxo-3-piperidyl)phthalimide had been developed by the company Grünenthal and was soon prescribed as a sleep-inducing drug and to combat symptoms associated with morning sickness of pregnant women. However, when taken during the first trimester of pregnancy, thalidomide prevented the proper growth of the foetus, resulting in horrific birth defects such as severe deformations of the vertebral column and the extremities.
This collection of links tries to present some of the most interesting web resources related to the subject and covers websites and documents written in English and German. Naturally, the list does not claim to be complete, but aims to serve as a starting point for all those who want to gather information on the subject.
I am not responsible for the contents of the listed external sites. Nevertheless, I have checked all links to the best of my knowledge before adding them to the list, to make sure that the presented medical and scientific information is correct and the linked pages do not contain any illegal or offending material. However, if you feel that a site should be removed from the list because the contents might have been changed by the site owner, do not hesitate to contact me. I will check the link and remove it, if necessary.
All pages and documents written in German are marked with the tag "[de]".
General Information
Comprehensive Articles
- Wikipedia Article about Thalidomide
- Clear and easily understood contribution with several cross-references.
- Onmeda: Contergan [de]
- Short Review of the Thalidomide and Contergan history.
- Contergan - ein Überblick [de]
- Short and fluently written summary touching on many aspects of the Contergan story. [PDF, 90KB]
- Clinical Toxicology Review
- The article covers several aspects such as clinical uses, mechanism of action, administration, dosage and pharmacokinetics, toxicity, and treatment of poisoning.
- Thalidomide
- General information including material on government regulation and safeguards, provided by the NTP Center for the Evaluation of Risks to Human Reproduction.
- Thalidomide product monograph
- Pharmion Information Brochure for health professionals in Australia. The document presents information on chemistry and clinical pharmacology of thalidomide, its use in Erythema Nodosum Leprosum and Multiple Myeloma, data about safety, tolerability, dosage and administration of the drug, the Pharmion Risk Management Programme (PRMP), and a list of references. [PDF, 1.52MB]
- Prescribing information for Thalomid Capsules (Celgene)
- The data sheet provides information and data on clinical pharmacology, indications and usage, contraindications, warnings and precautions, adverse reactions, dosage and administration, and storage and dispensing. [PDF, 86KB]
- Eine unendliche Geschichte [de]
- Recommendable review article by Prof. Klaus Roth. For several years it was unclear, whether any of the actions of racemic thalidomide could be separated out using a pure enantiomere. When, for example, given to rats and mice, (-)-(S)-thalidomide caused dose-dependent teratogenicity in both species, whereas (+)-(R)-thalidomide was devoid of this effect. However, these putative differences in therapeutic and adverse effects would to a large extent be abolished by a rapid interconversion in vivo of separately administered enantiomers. Thus, the use of pure (+)-(R)-thalidomide instead of the racemate would not have prevented the Contergan tragedy happening. [PDF, 560KB]
Brand Names
- The Many Faces of Thalidomide
- Comprehensive list of thalidomide's brand names in different countries.
Contergan and the company Grünenthal
Between 1955 and 1957 the chemical compound α-Phthalimidoglutarimide was developed at Grünenthal GmbH, Stolberg (Germany) under the supervision of the responsible chemist Dr. Heinrich Mückter. The new substance named thalidomide became the active agent of the calming and sleep-inducing drug Contergan that was introduced on to the market on October 1, 1957 in West Germany.
- Grünenthal GmbH [de] - Grünenthal Group
- Websites of the Grünenthal company, former manufacturer of Contergan. Astonishingly there is only this very short statement on Thalidomide available on the public pages covering the saddest chapter of company history.
- Chemie Grünenthal
- The company Chemie-Grünenthal manufactured thalidomide until the mid 1990s. Interesting figures regarding production and distribution of the substance for the period of 1985 to 1992.
Thalomid® (Thalidomide) on the International Markets
- Celgene Corporation To Expand Thalomid Franchise Internationally
- Celgen Corporation, the registration holder for Thalomid within the United States, has signed agreements for the international registration and commercialization of Thalomid with the UK based companies Pharmion Corporation and Penn Pharmaceutical Services.
Chemistry
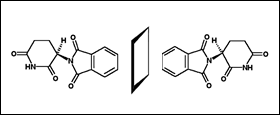
Thalidomide (α-phthalimidoglutarimide) has a chiral centre and was dispensed as a racemate (1:1 mixture) of dextrorotatory (R)- and levorotatory (S)-thalidomide. Initial animal studies indicated that the enantiomers have different biological properties: Calming and sleep-inducing effects are associated with the R-enantiomer, whereas teratogenic effects are more closely associated with the S-enantiomer. Under physiological conditions, both enantiomers undergo rapid interconversion making a total separation of their clinical effects unfeasible.
Synonyms
- Thalidomide
- α-Phthalimidoglutarimide
- (±)-N-(2,6-Dioxo-3-piperidyl)phthalimide
- (±)-N-(2,6-Dioxo-3-piperidinyl)-1H-isoindol-1,3(2H)-dione
Structural Formula
General Information Regarding Chemical and Physical Properties
- Data Sheet
- Information covering handling, storage, physicochemical properties, and toxicology. [PDF, 46KB]
Racemization of Thalidomide
- Stereospecific Determination, Chiral Inversion In Vitro and Pharmacokinetics in Humans of the Enantiomers of Thalidomide
- (R)- and (S)-thalidomide show rapid interconversion in vivo. The article provides pharmacokinetic data on chiral inversion and elimination of thalidomide enantiomers.
History
- Die Nachtseite des Wirtschaftswunders [de]
- In 1961 every third German took Contergan to get to sleep: Taking stock 40 years later. Newspaper article published in the FAZ, 2001-11-25.
Widukind Lenz
The German human geneticist Dr. Widukind Lenz (1919-1995) deserves the honour for being the first - apart from the Australian physician McBride - who recognized and published the connection between children's birth defects and the fact that their mothers had taken thalidomide during pregnancy.

- Widukind Lenz
- Biographic data, short outline of the thalidomide history, introduction of a lecture on thalidomide, and bibliographic information.
- The History of Thalidomide
- Extract from a lecture given by Dr. W Lenz at the 1992 UNITH Congress.
Frances Oldham Kelsey
Although industry put massive pressure on the FDA, the agency refused to approve the drug in the United States in 1960. It was Frances Oldham Kelsey, the agency's newest reviewer, who probably prevented a disaster by her refusal to clear thalidomide without satisfactory documentation regarding the side effects of the substance.
- Frances Oldham Kelsey: FDA Medical Reviewer Leaves Her Mark on History
- Comprehensive biographical acknowledgement of Kelsey's great contribution to drug safety and modern drug regulation.
The Thalidomide Disaster
The thalidomide disaster has had a lasting effect on drug research and drug regulation. A bitter lesson with far-reaching consequences.
- Die Contergan-Katastrophe revisited [de]
- Contribution of Hans-Jochen Luhmann to the discussion about the root cause of the Contergan disaster in Germany and the question why it has not been avoided. [PDF, 141KB]
- A Tale of Two Experts: Thalidomide and Political Engagement in the United States and West Germany
- Different political conditions in the United States and Germany resulted in very different situations regarding the risk assessment and clearance for the sale of thalidomide containing drugs. [PDF, 112KB]
- Die Rolle der Ärzteschaft bei der Aufklärung der Contergannebenwirkungen und die Auswirkung auf die deutsche Arzneimittelgesetzgebung [de]
- Lecture given at the symposium "Die Contergankatastrophe - Eine Bilanz nach 40 Jahren", organized by the Deutsches Orthopädisches Geschichts- und Forschungsmuseum e.V. (Frankfurt Main, 2003). [PDF, 67KB]
The Contergan Lawsuit
On January 18, 1968 the Contergan case was brought to trial at the criminal division of the Aachen regional court. The owner of the company and responsible employees of the Grünenthal GmbH were accused of causing deliberate injury and death resulting from negligence. Three years later, on December 18, 1970, the court dismissed the case due to only minor responsibility of the accused and "minor importance to the public of the Federal Republic of Germany". A victory of the pharmaceutical lobby over the interests of the affected victims?
- Contergan - eine Kurzdarstellung der Ereignisse [de]
- Summary of the lawsuit. The article adopts a clear position in favour of the thalidomide victims.
- Der Prozeßbetrug [de]
- Interview with Peter Plichta, pharmacist and private scholar, who shows the case in the light of a conspiracy theory.
Medicinal Aspects
Active Principle
For several years there seems to be a general consensus that the teratogenic effects of thalidomide are caused by the fact that the substance is strangulating the blood supply to the embryo's limbs. This antiangiogenic effect turns Thalidomide into an interesting agent for fighting cancer as the tumor growth can be affected by inhibition of its vasculogenesis. There are also promising reports on positive effects of thalidomide in treatment of HIV and leprosy.
Correlation between Thalidomide Malformation and Phase of Pregnancy
When thalidomide is taken between day 35 and 50 of pregnancy there is a high risk of dysmelia (deformation of extremities).
- Fehlbildungszeitplan bei Thalidomid-Einwirkung [de]
- Presentation in tabular form on the pages of the Karlsruher Conterganverband e.V. There is also a diagram available [155KB].
New Therapeutic Approaches
General Information
- Phönix aus der Asche? [de]
- Currently thalidomide makes an astonishing comeback. Once banned for its teratogenic side effects the drug is now used successfully for treating cancer, HIV and leprosis. Newspaper article by Klaus Koch, published in "Die Woche", 1996-04-12.
- Das stille Comeback eines totgeglaubten Medikaments [de]
- Thalidomide on its way from the Contergan disaster to a promise for incurable patients. Newspaper article about new fields of application for an old drug, published in the NZZ, 2003-11-05.
- Dem Tumor den Saft abdrehen [de]
- The terrible drug thalidomide, once responsible for causing severe birth defects in thousands of unborn children, is experiencing a comeback as a cancer treatment. Pharmaceutical companies sense the opportunity for big business. Article from the German weekly journal "Der Spiegel" (43/2004) about the comeback of thalidomide in cancer therapy.
- Vom Horrormedikament zum Wundermittel [de]
- Thalidomide shows promising results in the treatment of several diseases, especially leprosy and cancer. Article published in the scientific section of the German newspaper "Die Welt", 2005-04-05.
- Der Contergan-Wirkstoff wird zum Hoffnungsträger [de]
- Interview with Prof. Dr. Kurt Eger, Professor of Pharmaceutical Chemistry at the University of Leipzig.
- Reinventing thalidomide
- Easy-to-understand article from chembytes e-zine. Based on a short historical retrospect, the author presents the different effects and therapeutic uses of thalidomide. The clear explanation of the drug's antiangiogenetic activity through its interaction with DNA is of particular interest.
Review Articles
- A review of thalidomide's history and current dermatological applications
- In 1998, thalidomide became FDA approved for the acute treatment and suppression of the cutaneous manifestations of erythema nodosum leprosum (ENL). Off-label uses for thalidomide include: aphthous stomatitis, Behçet disease, pyoderma gangrenosum, chronic discoid lupus erythematosus, systemic lupus erythematosus, lichen planus, prurigo nodularis and sarcoidosis. This review from the Dermatology Online Journal examines the background, pharmacokinetics, mechanism of action, side-effects, and indications of thalidomide.
- Thalidomide: from tragedy to promise
- Peer reviewed article, published in Swiss Medical Weekly, which summarises the most relevant clinical studies currently performed with thalidomide. [PDF, 220KB]
- Thalidomide: Tragic Past and Promising Future
- This comment, taken from the Mayo Clinic Proceedings gives an overview of thalidomide history, its reintroduction into clinical practice, and future directions. With many references. [PDF, 71KB]
Mechanism of Action
- Antiangionetic Activity of Thalidomide
- Orally administered thalidomide is an inhibitor of angiogenesis as shown by this scientific paper.
- Angiogenesehemmer als Krebsprophylaxe [de]
- The relatively new class of angiogenesis inhibitors blocks the formation of new blood vessels, which are essential for tumor growth. In the future these substances might be used as preventive therapy for patients with known tumor risk. Short journal article, published in the PZ.
- A Water-Soluble Analog of Thalidomide
- Structural modifications of thalidomide have been designed which appear to be nontoxic, nonmutagenic, and nonteratogenic. Scientific article from The Journal of Immunology, published in 1998. There is also a PDF Version [211 KB] available.
- Können Radikalfänger dem Thalidomid zu einem Comeback verhelfen? [de]
- The teratogenic effect of thalidomide is, among other things, caused by radical induced oxidation of embryonic macromolecules such as DNA. The use of free radical catcher might possibly prevent this severe side effect. Short journal article, published in the PZ.
Treatment of Multiple Myeloma
- Antitumor Activity of Thalidomide in Refractory Multiple Myeloma
- Thalidomide can induce marked and durable responses in some patients with multiple myeloma, including those who relapse after high-dose chemotherapy. Results of a clinical study.
- Thalidomide in Myeloma Treatment
- Comprehensive description of thalidomide's antimyeloma activity including detailed information on its mechanism of action, dosage and administration, and clinical trials.
- Thalidomide for Patients With Relapsed Multiple Myeloma After High-Dose Chemotherapy and Stem Cell Transplantation
- Original paper, recently published in Mayo Clinic Proceedings. The article contains an interesting figure illustrating the pathways of thalidomide activity against multiple myeloma. [PDF, 109KB]
- Thalidomid und andere Therapieansätze [de]
- Successful treatment of resistant and relapsing multiple myeloma with thalidomide: overview of therapeutic approach and clinical results. [PDF, 140KB]
- Guideline: Thalidomide in Multiple Myeloma - Current Status and Future Prospects
- Paper of the British Committee for Standards in Haematology (BCSH) giving recommendations for the use of thalidomide in treatment of patients with refractory or relapsed myeloma, published in the British Journal of Haematology, 2003, 120, 18-26. [PDF, 92KB]
- Empfehlungen zur Anwendung von Thalidomid bei Patienten mit einem multiplen Myelom [de]
- Based on the available literature and scientific data the German Society for Haematology and Oncology DGHO has provided a consensus paper giving a guideline regarding the use of thalidomide for the treatment of relapsed/refractory myeloma. [PDF, 307KB]
- Ein vor 40 Jahren verstoßenes Medikament weckt neue Hoffnungen [de]
- Thalidomide, which harmed more than 10,000 children 40 years ago, is now having an astonishing come-back as therapeutic agent against multiple myeloma. Report of a concerned patient. [PDF, 17KB]
Treatment of Leprosis
- Use of thalidomide in leprosy
- Scientific article by C. L. Crawford (published in 1994) which takes a critical view and comes to the conclusion that thalidomide should not be used any longer for the management of erythema nodosum leprosum.
The Second Chance
- Thalidomide Information
- The FDA has approved the use of thalidomide for the treatment of leprosy. This drug info page presents consumer and patient information, thalidomide advisory committee and workshop transcripts, the approved labeling text and the medical review on which the decision to approve this drug was based. Selected links to other web sites containing information on thalidomide are also included.
- Thalidomide: Potential Benefits and Risks
- Program and abstracts of an open public scientific workshop, sponsored by by the National Institutes of Health and the Food and Drug Administration, September 9-10, 1997, Bethesda, MD. The workshop was designed to assess the benefits and risks and to guide further investigation and clinical use of thalidomide. [PDF, 548KB] - There is also an executive summary [HTML] available.
- Thalidomide Pharmion Approved in Turkey
- Following the United States, Australia, New Zealand and Turkey have now also approved thalidomide as maintenance therapy for prevention and suppression of cutaneous manifestations of erythema nodosum leprosum (ENL) recurrence and treatment of multiple myeloma after failure of standard therapies. Pharmion press release.
- Thalidomid vor der Wiederzulassung? [de]
- In the course of the registration procedure EMEA officials met representatives of thalidomide victims from Great Britain, Sweden and Germany as part of their consultation process. Newspaper article published in the FAZ, 2003-01-29.
- Patients denounce the withdrawal of the marketing authorisation application
- Pharmion Corporation's decision of May 19, 2004 to withdraw their marketing authorization application for thalidomide is criticized by multiple myeloma patient's representatives. EURODIS press release, 2004-07-08. [PDF, 66KB]
- Bekanntmachung zu Thalidomid-haltigen Arzneimitteln [de]
- Joint statement of the German Bundesinstitut für Arzneimittel und Medizinprodukte, Arzneimittelkommission der Deutschen Ärzteschaft and Arzneimittelkommission der Deutschen Apotheker (2003-12-22). The notice gives guidance on legal aspects regarding the use of thalidomide, as the EMEA hasn't approved it for the treatment of multiple myeloma and erythema nodosum leprosum yet.
Scientific Bibliography
There is an enormous number of scientific papers dealing with thalidomide and the Contergan disaster. The following bibliographies provide an overview of the relevant publications:
- Thalidomide: Potential Benefits and Risks
- This extensive bibliography, provided by the National Library of Medicine, is divided into different thematic sections and covers the period from January 1963 until July 1997. Most of the papers cited are written in English.
- Keyword Search for "Thalidomide"
- Result of an up-to-date search for the keyword "Thalidomide" within the online database of the National Library of Medicine. The search link also provides reference to recent documents published later than 1997. Abstracts of most cited papers available.
- Keyword Search "Contergan"
- Result of an up-to-date search for the keyword "Contergan" within the online database of the National Library of Medicine. The search link also provides reference to recent documents published later than 1997. Abstracts of most cited papers available.
Support and Advocacy Groups and Organizations
On October 01, 1957 the first thalidomide containing drug entered the market (Contergan in Germany). In the course of the next four years more than 10,000 so called "Thalidomide babies", children with severe birth defects such as phocomelia, disfigurement, deafness, blindness, and other internal disabilities were born worldwide. Finally, though much too late, Contergan was banned from the German market in November 1961, and other countries followed soon after. There are approximately 5,000 survivors alive today, around the world. These "Thalidomiders" (as they call themselves) have founded support and advocacy groups to enforce their social and political claims.
- Bundesverband Contergangeschädigter [de]
- Presentation of the German Association of Thalidomiders with mission statement, schedule, press releases, and information on the historic background.
- Hilfswerk für Contergangeschädigte E.V. Hamburg [de]
- The Hamburg based local branch of German Thalidomiders provides on its website extensive information on the subject and interesting links.
- Karlsruher Conterganverband e.V. [de]
- Website of the Karlsruhe section of German Thalidomiders with information on the historical and medical background of the Contergan disaster, activities, and lobbying.
- Interessenverband Körpergeschädigter e.V. - Contergangeschädigten Hilfswerk [de]
- The review on the documentary film "Contergan: Die Eltern" is worth reading.
- thalidomide.org
- Website of the Thalidomide Association of Sweden. The pages listed under the menu item "Thalidomide" are very informative, in particular Against Leprosy, which describes the use of thalidomide in treatment of leprosy.
- Thalidomide Victims Association of Canada
- Good website covering many aspects of the subject. The menu items "information" and "links" provide several recommendable references about the current use of thalidomide and malformations caused by this drug.
More
- Grünenthals vergessene Kinder [de]
- Even more than 40 years after the Contergan disaster, the trust fund established by the Federal Republic of Germany and the pharmaceutical company Grünenthal for the financial compensation of victims is still receiving applications for compensation although the deadline was December 31, 1983.
- "Seid ihr alle krank?" [de]
- Interview with the world-famous singer and Contergan victim Thomas Quasthoff, who talks about his physical handicaps and his life as a singer.
- Deutsche Geschichten - Anne [de]
- Interview with a woman who is now 70 years old and took Contergan in 1961 but nonetheless gave birth to a healthy boy.
- Zwei Seiten eines Medikaments [de]
- Thalidomide is a striking example of how the image and mirror image forms ("enantiomers") of a drug can have totally different effects. Descriptive information about chiral drugs taken from the feature story "Das Rätsel von links und rechts" of the German television series "Quarks & Co.".
- Stereochemistry: an introduction
- Tutorial presenting the basics of stereochemistry in a clear and simple way. [PDF, 248KB]
- Höhere Arzneimittelsicherheit für Schwangere [de]
- Even though 42 years have passed since the Contergan disaster there is still not sufficient information available regarding the use of drugs during pregnancy. As of January 01, 2004 the Berlin Informationszentrum für Embryonaltoxikologie started to collect data on side effects of drugs during pregnancy and lactation. It is the first time ever such a survey is performed methodically and Germany-wide.
URL: http://www.k-faktor.com/thalidomide/ | Last Update: 06.04.2007
cantor's homepage - © Jürgen Klein 2002-2008